Research
... the clinical testing and introduction of new treatments and optimal rehabilitation for spinal cord injury.

Ongoing Clinical Trials
The following clinical trials are currently ongoing:
Nogo Antibody Study
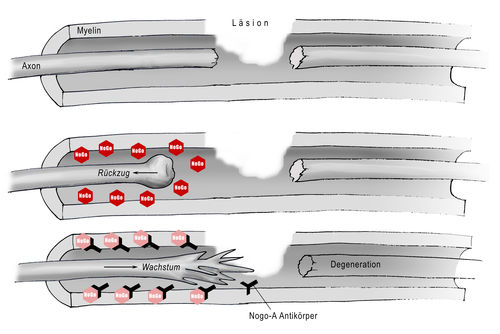
The study aims to prove that Nogo antibodies can make damaged nerve fibres regrow in humans (not just animals) and that this growth can lead to partial improvement of the bodys functions and sensitivity. A Phase I safety study of the anti-Nogo-A antibody trial in freshly injured paraplegic patients started in summer 2006. A placebo controlled Phase 2 “proof of concept study” is in preparation. The Nogo antibody study is being conducted in Switzerland in close cooperation between the Paraplegic Centre at Balgrist University Hospital, various trauma centres and Novartis. Read more...
RANA
Robotics and related technologies have the potential to facilitate neurorehabilitation therapy. At the Sensory-Motor Systems Lab ETH, Zurich, in collaboration with the University Hospital Balgrist, Zurich, a robot for rehabilitation of the arm was developed. ARMin III is an exoskeleton robot with seven degrees of freedom. In combination with virtual reality applications it allows for task-oriented adaptive training adjusted to each single patients need. It is used for research and evaluated in patients suffering from stroke.
In the RANA project we are developing novel methods of assessment that are automated, objective and reproducible. The focus of the RANA project is to i) to derive novel functions for robot-assisted automatic assessment and Activities of Daily Living (ADL) training, ii) to introduce and evaluate the effectiveness of these novel functions for rehabilitation in SCI patients and iii) to perform an collaborative effort with the industrial partner Hocoma AG, Volketswil, Switzerland, to transfer technology and clinical experience into commercialization.

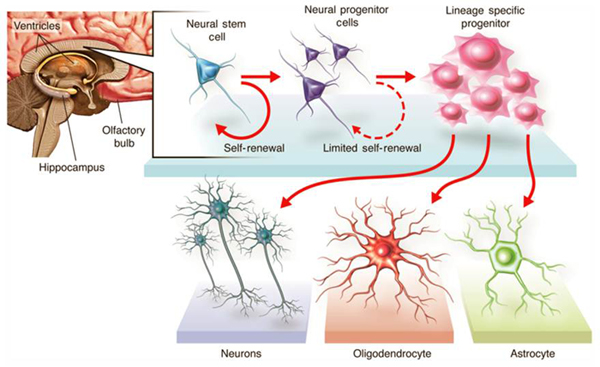
Neural Stem Cell Trial
The trial did enroll 12 patients with thoracic (chest-level) spinal cord injury who have a neurological injury level of T2-T11, and included both complete and incomplete injuries as classified by the American Spinal Injury Association (ASIA) Impairment Scale. The first cohort were patients classified as ASIA A, or patients who have what is considered to be a "complete" injury, or no movement or feeling below the level of the injury. The second cohort did progress to patients classified as ASIA B, or patients with some degree of feeling below the injury. The third cohort consisted of patients classified as ASIA C, or patients with some degree of movement below the injury. In addition to assessing safety, the trial did measure defined clinical endpoints, such as changes in sensation, motor, and bowel/bladder function. All patients received HuCNS-SC cells through direct transplantation into the spinal cord, and were temporarily immunosuppressed. Following transplantation, the patients were evaluated regularly over a 12-month period in order to monitor and evaluate the safety and tolerability of the HuCNS-SC cells, the surgery and the immunosuppression, and to measure any recovery of neurological function below the injury site. As the Company intends to follow the effects of this therapy long-term, a separate four-year observational study was initiated at the conclusion of this trial.
The trial is initiated by StemCells, Inc. Palo Alto, California, and is being conducted at the Balgrist University Hospital, University of Zurich, Switzerland.
For further information: StemCells, Inc.